
Cobalt Oxide Hmoov Dub Co3O4 Hmoov
Product Description
Co3O4 yog dub lossis grey-dub hmoov.Qhov ceev yog 0.5-1.5g / cm3, thiab kais ceev yog 2.0-3.0g / cm3.Cobalt tetroxide tuaj yeem maj mam yaj hauv kub sulfuric acid, tab sis insoluble hauv dej, nitric acid thiab hydrochloric acid nyob rau hauv chav tsev kub.Thaum rhuab mus rau saum 1200 ℃, nws yuav decompose rau hauv cobalt oxide.Thaum rhuab mus rau 900 ° C nyob rau hauv ib tug hydrogen nplaim, nws yog txo mus rau hlau cobalt.
Cobalt oxide hmoov muaj cov yam ntxwv ntawm cov ntsiab lus me me, cov khoom siv tsis zoo, cov khoom siv tsis zoo, thiab lwm yam ntawm cov khoom siv hluav taws xob , thiab tuaj yeem siv dav hauv hluav taws xob, tshuaj lom neeg thiab cov khoom siv alloy.
Specification
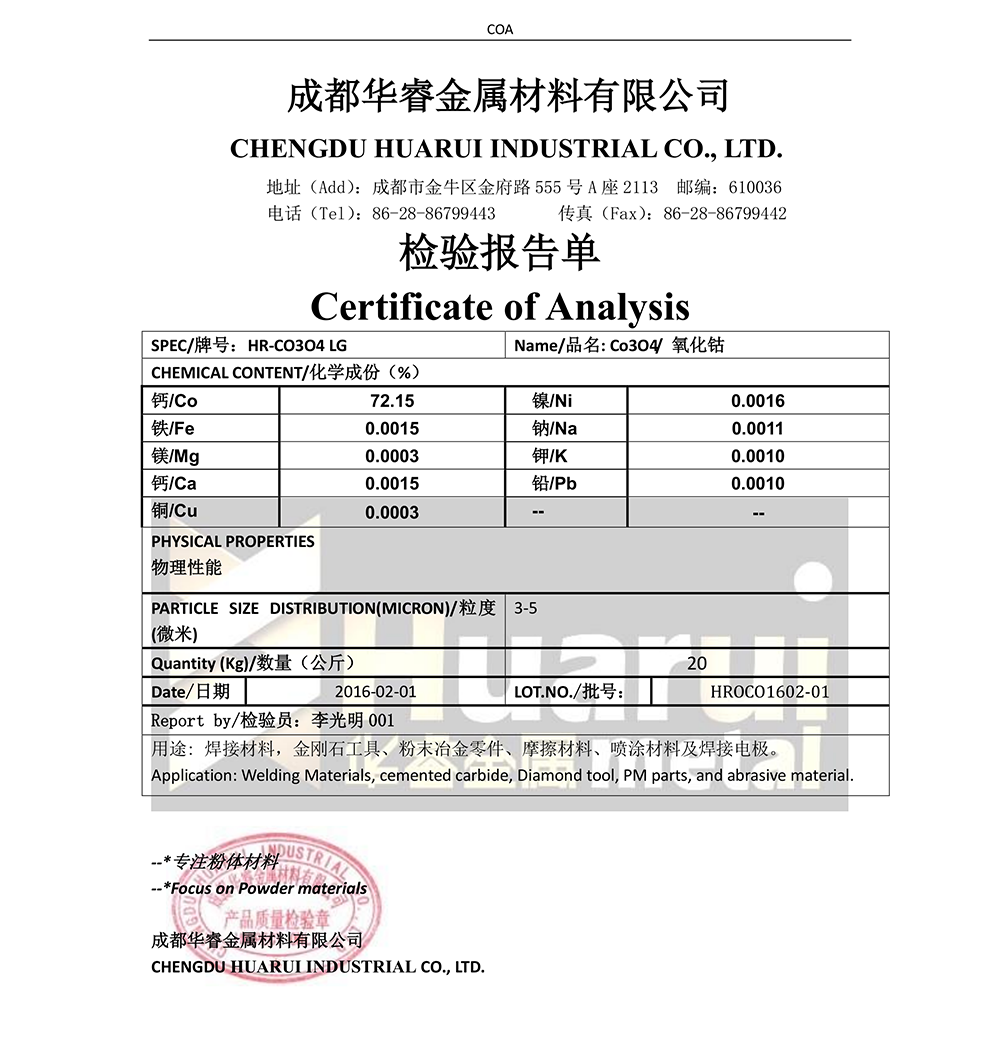
| Cobalt oxide hmoov muaj pes tsawg leeg | ||||||
| Qib | Muaj impurity (wt% max) | |||||
| Cov% | Ni% | Cu% | Mn% | Zn% | Fe% | |
| A | 73.5 ± 0.5 | ≤ 0.05 | ≤ 0.003 | ≤ 0.005 | ≤ 0.005 | ≤ 0.01 |
| B | ≥74.0 | ≤ 0.05 | ≤ 0.05 | ≤ 0.05 | ≤ 0.05 | ≤0.1 |
| C | ≥72.0 | ≤ 0.15 | ≤ 0.10 | ≤ 0.10 | ≤ 0.10 | ≤0.2 |
COA
Daim ntawv thov
1. Siv los ua xim thiab xim rau iav thiab ceramics, nyuaj alloy;
2. Oxidants thiab catalysts hauv kev lag luam tshuaj;
3. Siv hauv kev lag luam semiconductor, hluav taws xob ceramics, lithium ion roj teeb cathode cov ntaub ntawv, cov khoom sib nqus, ntsuas kub thiab roj;
4. Siv raws li siab purity analytical reagent, cobalt oxide thiab cobalt ntsev npaj
Kev tswj hwm qhov system

Huarui muaj kev tswj xyuas zoo.Peb sim peb cov khoom ua ntej tom qab peb ua tiav peb cov khoom, thiab peb sim dua ua ntej txhua qhov khoom xa tuaj, txawm tias cov qauv.Thiab yog tias koj xav tau, peb xav lees txais cov neeg thib peb los kuaj.Tau kawg yog tias koj nyiam, peb tuaj yeem muab cov qauv rau koj los kuaj.
Peb cov khoom zoo yog lav los ntawm Sichuan Metallurgical Institute thiab Guangzhou lub koom haum ntawm Kev Tshawb Fawb Hlau.Kev koom tes ntev nrog lawv tuaj yeem txuag tau ntau lub sijhawm sim rau cov neeg siv khoom.











